LabWare is the global leader in laboratory information management systems (LIMS), with over 3,000 customers including NIH, USDA, GSK, Pfizer, Hershey, Caterpillar, and Chevron and 98% customer satisfaction. For 30+ years, our laboratory automation platform has been ensuring data integrity/compliance, accurate test results, and more efficient throughput. Laboratories can choose from the cost-optimized + fully validated LabWare SaaS LIMS or the industry-optimized + fully customizable LabWare LIMS/ELN.
LabWare LIMS
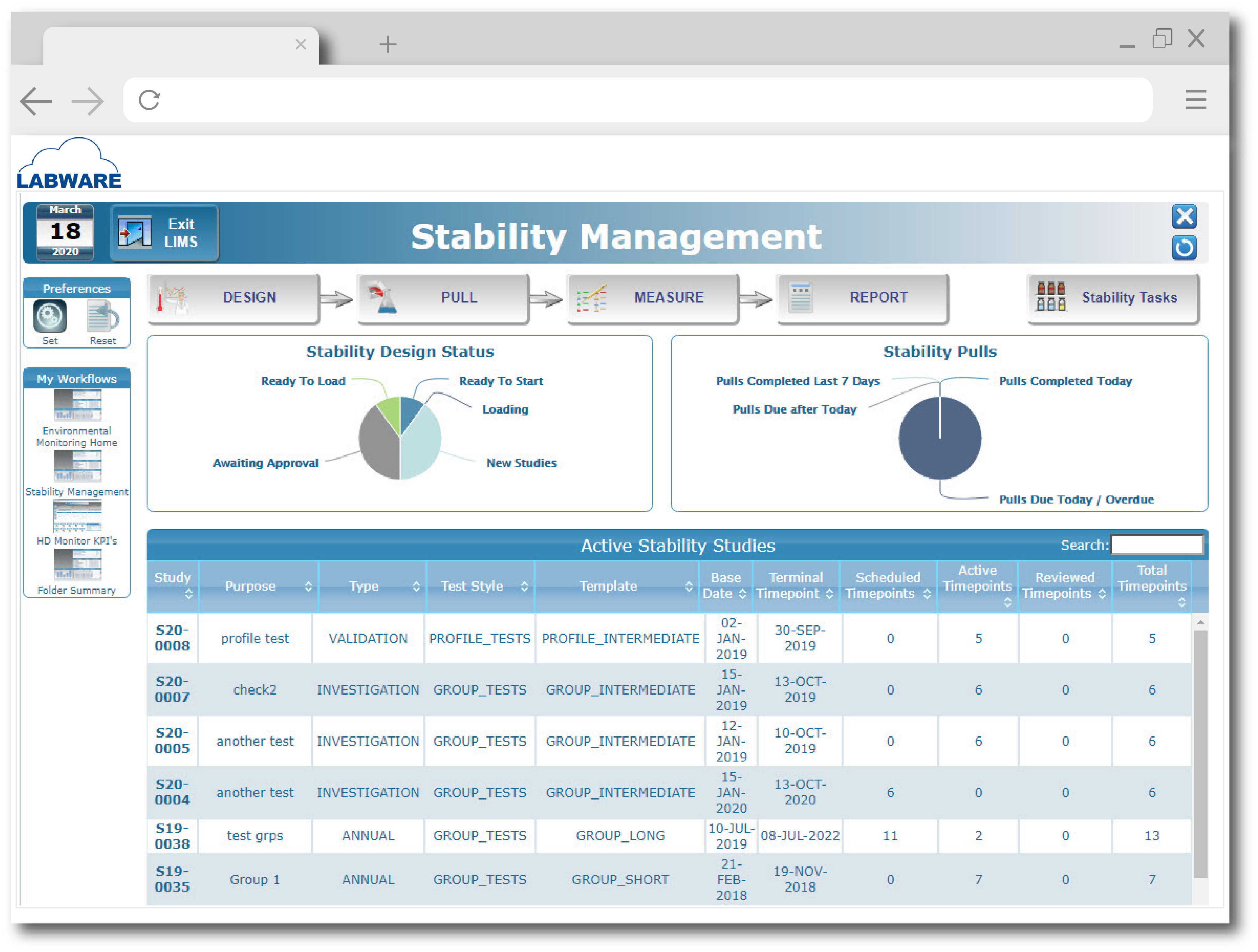
Images
Check Software Images




Customer Reviews
LabWare LIMS Reviews
Filippo G.
Advanced user of LabWare LIMSWhat do you like best?
the stability, flexibility, functionality, after pass the LW formation training, all system become really friendly and accessible. One big advance as well is the constant availability of different country support structure, that in timely and accurate manner solve questions and problematics, either by remote support either by remote connections.
What do you dislike?
after years and different experience in different situation, i did not find anything to dislike, with opportune tuning, it fully satisfy all needs that come out. On version 8th looks like also it has been improved the appearance that in previous version were still in 90 style. if we would be forced to find an area of improving, the only point of focus could be the use of crystal report, that on my opinion it need to be modified for allow user to generate report in a more friendly way
Recommendations to others considering the product:
I do consider LLW LIMS a real valid support for all day by day needs of Laboratory, suggesting to company to extend the use of this tool in all our subsidiary. Therefore it became natural to recommend to any other to app ly LW LIMS inside their laboratory if they was safety, security good data management in a easy and professional way. Any case i suggest to make an effort and install a full suite, including statistical packages and ISO 17025 adds
What problems are you solving with the product? What benefits have you realized?
Beside the standard laboratory use of the lims, in pair of occasions system solve some strong needs, some companies where worked, was not available any system for monitoring the employees presence and for schedule presences (holydays, courses...) with opportune modifications and arrangement with LW lims we were able to arrange an internal administrative system for personnel and timesheet generations.
Great benefit was an easy way to collect and organize data of laboratories, either coming form lab instruments either from on line analyzer. Another big advantage is the easy way to share and receive data with many external systems. Optimization of chemicals and spares parts is another important function that allowed me to safe money and avoid to duplicate purchases. Implementing the ISO 17025 controlling package, allowed me to obtain the laboratory accreditation in most easy way and provide a set of control for better validate the results published. In some companies where i worked it was difficult to have available an official way for monitoring and manage various report and study realized from laboratory, in this case LW LIMS come in our help providing the Document Management System, an internal functionality that allow to control and verify the life cycle of any document initiate inside this patch, from the first issuing, revision and final authorizations, all stages are monitored and consultable. Usage of this DMS and patch for document automatic compilation make faster all reports generations inside the laboratory, maintain in any case a strong control on document quality.
Las but not least using the auditing internal system, we can have the complete control of all LIMS environment, verify and assuring that all action done on sample or any other lims aspect are monitored and controlled, leaving the system in a really safety and sure situation.














