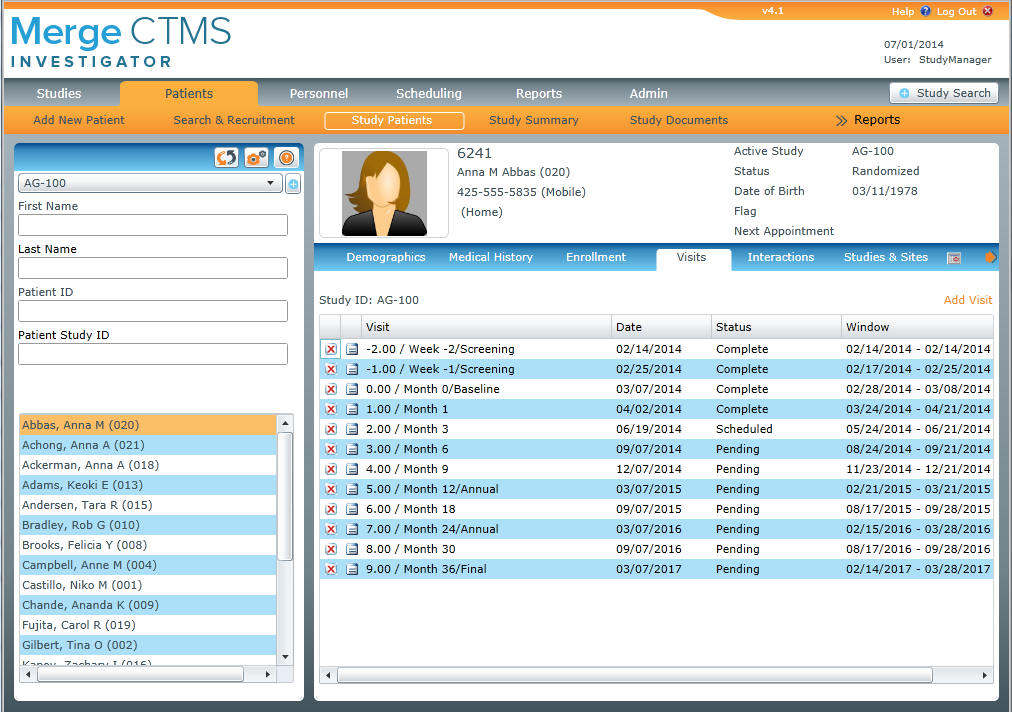
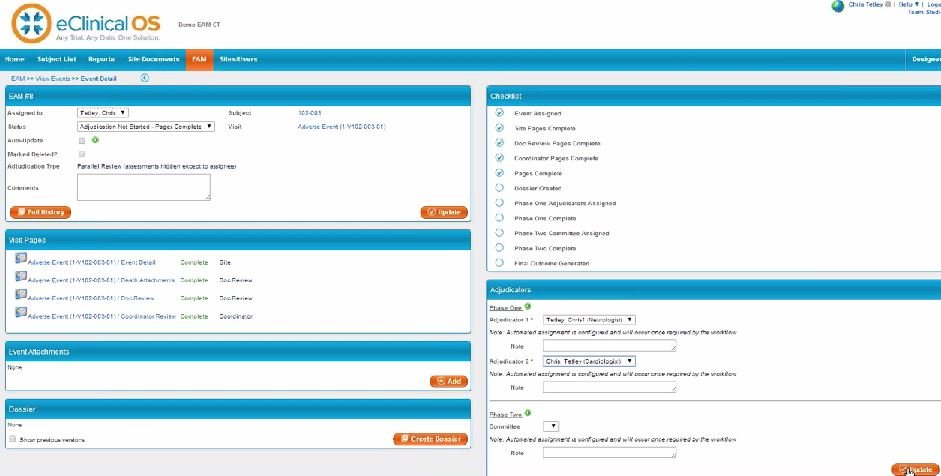
With a robust electronic data capture (EDC) system at its core, IBMВ® Clinical Development is an all-in-one, cloud-based data management platform that lets you design and manage clinical trials with more control, convenience and confidence than ever before.
IBM Clinical Development offers a host of powerful tools—from randomization, dispensing and reporting to endpoint adjudication and ePRO (electronic patient reported outcomes)—that help you launch and complete studies more efficiently and bring needed products to patients sooner.
Used by clinical professionals worldwide, the platform lets you capture, manage, analyze and report study data across therapeutic areas, trial types and time zones.
A SaaS solution, IBM Clinical Development requires no special infrastructure or prior programming experience to use. Designed to be intuitive and easy to navigate, you access all study data and platform functions through a centralized, password-protected web interface.