ResearchManager developed three eClinical tools: EDC, CTMS & ERMS
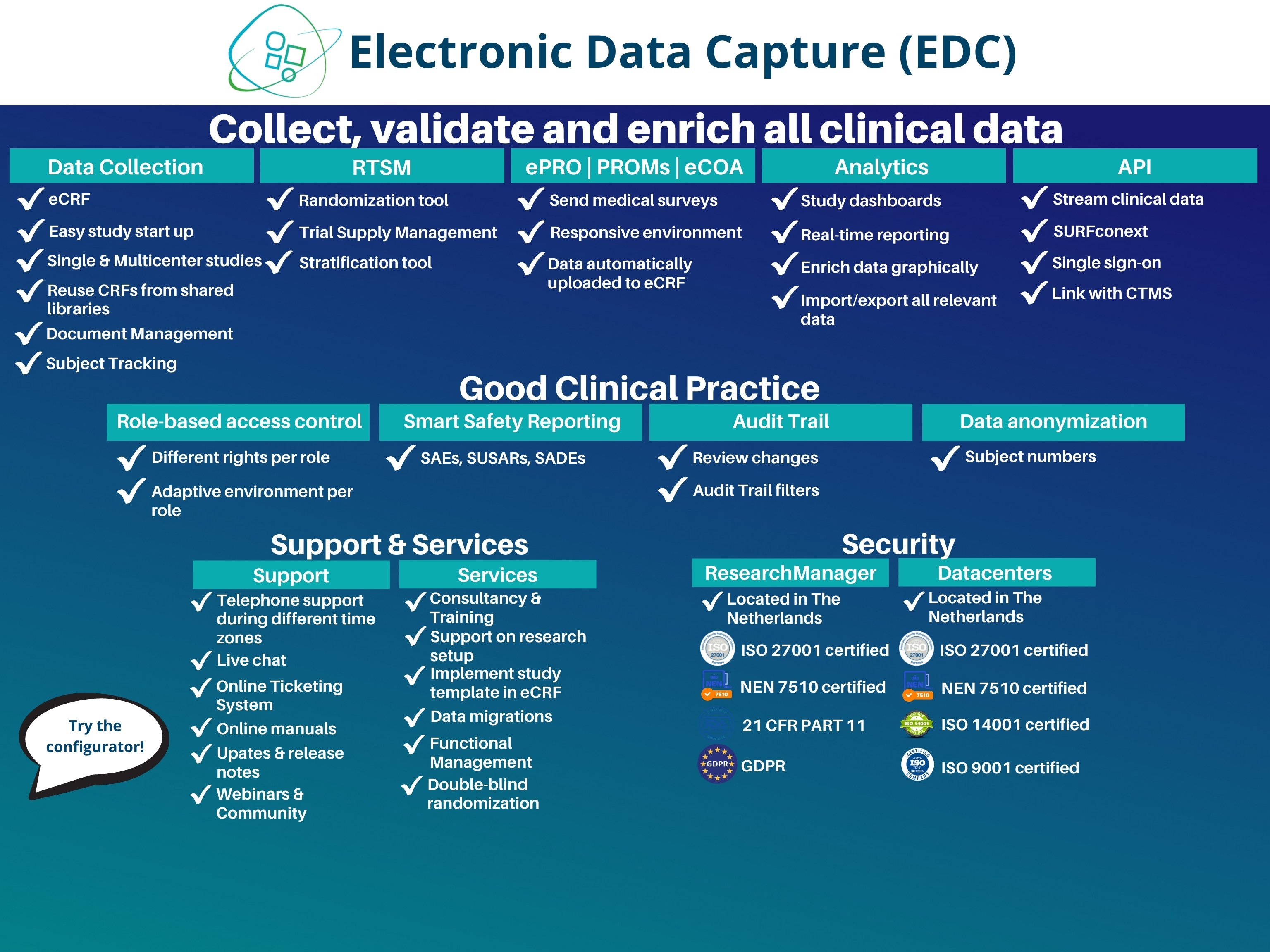
в—ЏElectronic Data Capture (EDC): collect, validate and enrich data under the 21 CFR Part 11, GCP & GDPR guidelines.
Try our EDC configurator вћћ https://form.jotform.com/211124166785354
в—ЏClinical Trial Management System (CTMS): effectively manage and track the entire study start-up & portfolio
For more informationвћћ https://my-researchmanager.com/en/prijzen-study-management-en/
в—ЏEthical Review Management System (ERMS): Medical Ethical Committees can review & manage all clinical study protocols in just one environment.
For more information вћћ https://my-researchmanager.com/en/prijzen-metc-en/
References:
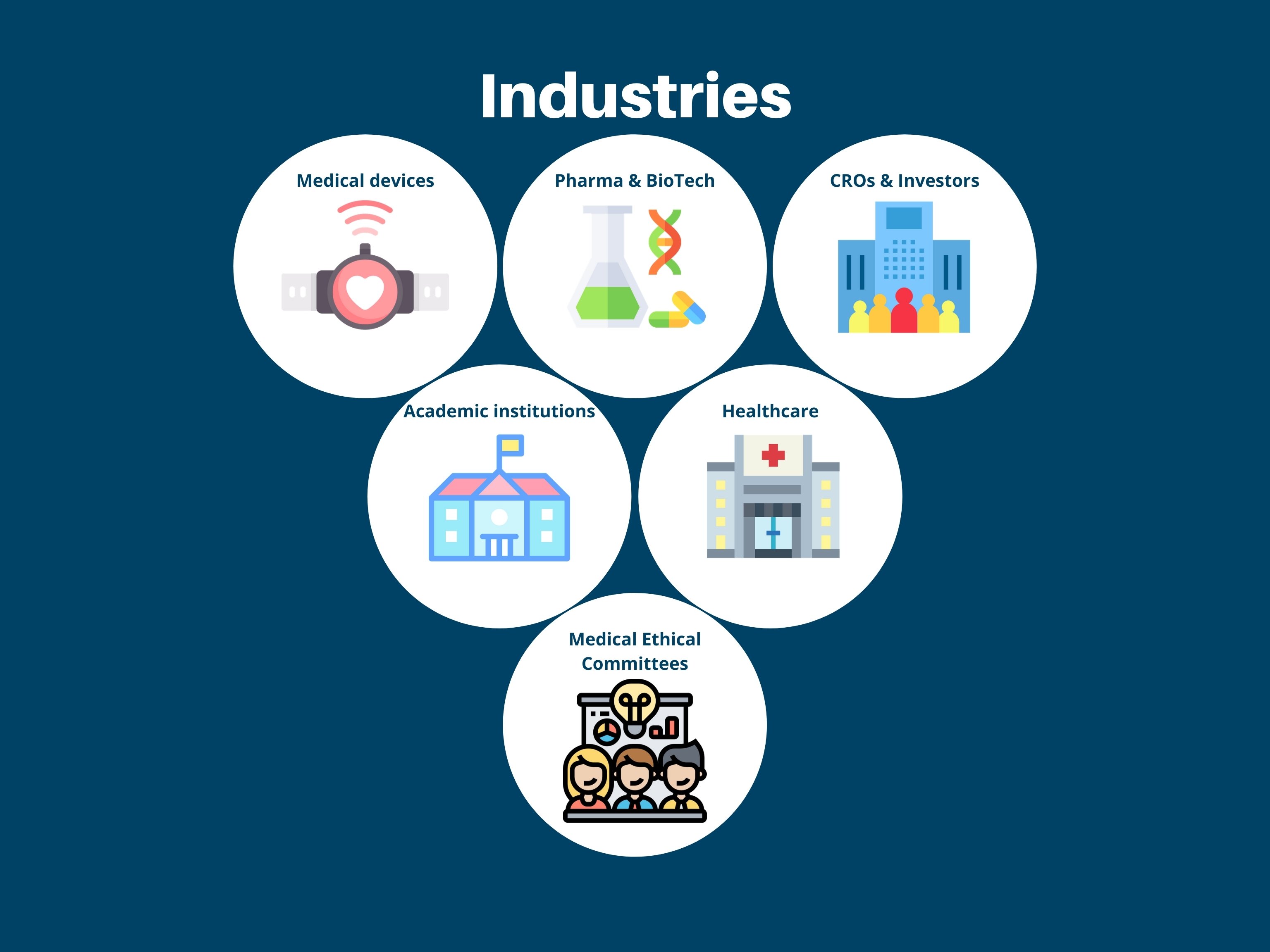
We have references in various sectors, such as: Medical Devices, Pharma & BioTech, CROs & Investors, Aademic institutions, Healthcare and Medical Ethical Committees.
Book a demonstration:
вћћ https://my-researchmanager.com/en/demorequest/